
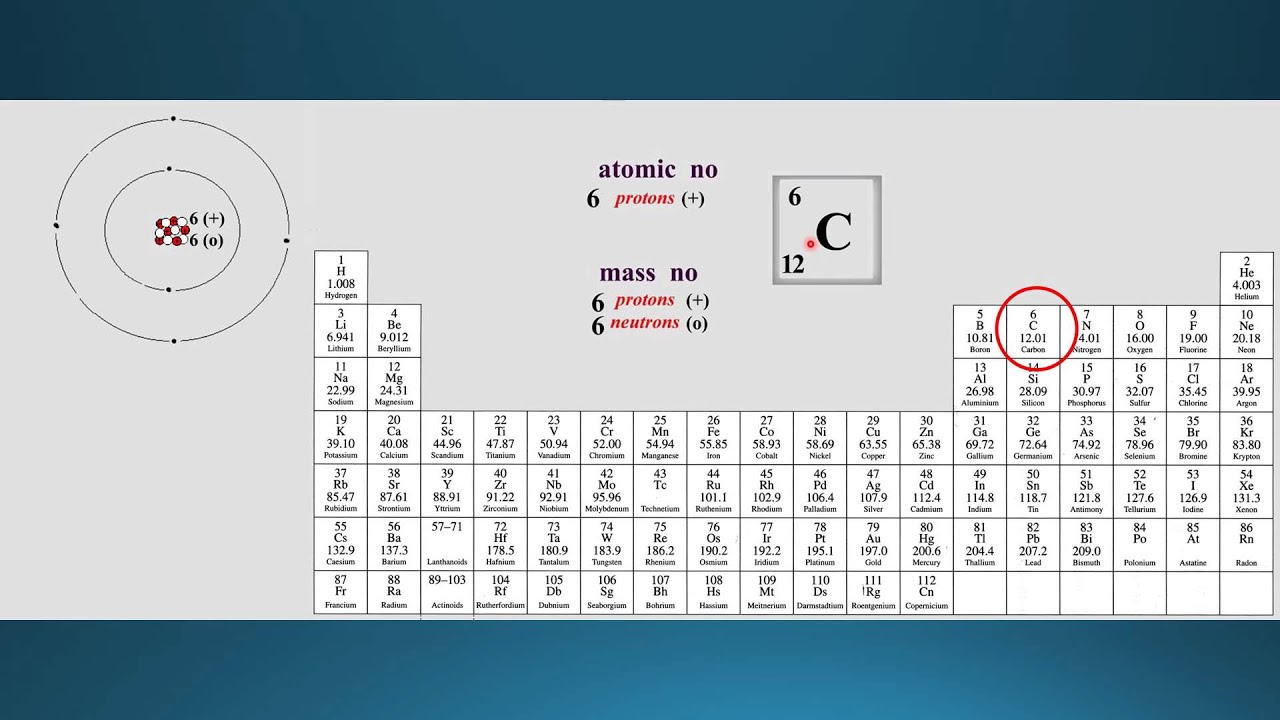

Each element has a unique atomic number, which indicates how many protons it has. The elements are arranged in order of increasing atomic number. Let’s start with the most obvious arrangement – the atomic number. What’s the best way to look at the periodic table? In devising the table, Mendeleev drew on the work of John Dalton, one of the first scientists to determine the atomic mass of elements, and of Johann Wolfgang Döbereiner, on trends in certain properties of selected groups of elements. Many of the elements known today were not discovered in Mendeleev’s time, and the information available on the known ones was limited. He relied on all the information available in 1869 about the known elements, and looked for a way to contain all the knowledge of these elements and set it out before the readers by the very form of the table.
PERIODIC TABLE WITH ATOMIC NUMBER AND ATOMIC MASS HOW TO
To be able to appreciate its genius and glean the wealth of information that it holds – you need to know how to read it.ĭmitri Mendeleev, who developed it, organized the elements in a way that captured their properties. They may think it is nothing more than a way of organizing the natural elements. Many people do not understand the importance of the periodic table of elements.


 0 kommentar(er)
0 kommentar(er)
